RASPA: Molecular Simulation Software for Adsorption and Diffusion in Flexible Nanoporous Materials
Introduction
RASPA is a software package for simulating adsorption and diffusion of molecules in flexible nanoporous materials. The code implements the latest state-of-the-art algorithms for Molecular Dynamics and Monte Carlo in various ensembles including symplectic/measure-preserving integrators, Ewald summation, Configurational-Bias Monte Carlo, Continuous Fractional Component Monte Carlo, Reactive Monte Carlo, and Baker’s minimization. Applications of RASPA include computing coexistence properties, adsorption isotherms for single and multiple components, self- and collective diffusivities, reaction systems, and visualization.
After 10 years of development, the software is now released under the GNU General Public License in 2016 (D. Dubbeldam, S. Calero, D.E. Ellis, R.Q. Snurr, Mol. Simulat. 2016). The package is now in use by over 30 research groups and institutions, including groups at the University of Amsterdam, Delft University of Technology, Georgia Tech (Atlanta, USA), Northwestern University (Evanston, USA), Shell (Amsterdam), and CSIRO (Melbourne, Australia).
Credits: RASPA has been created by David Dubbeldam (University of Amsterdam, The Netherlands), Sofia Calero (Eindhoven Technical University, The Netherlands), Thijs Vlugt (Delft University of Technology, The Netherlands), and Randall Q. Snurr (Northwestern University, Evanston, USA).
The source code can be obtained from github https://github.com/iraspa/raspa2. RASPA is provided without any kind of support or warranty. It should be viewed as an educational research-code that could be useful for researchers working in the field. Note, there is no support for the code.
Force fields implemented in RASPA for Zeolites
-
 Argon in Zeolites https://doi.org/10.1021/jp803753h
Argon in Zeolites https://doi.org/10.1021/jp803753h"Unraveling the Argon Adsorption Processes in MFI-Type Zeolite", E. García-Pérez, J. B. Parra, C. O. Ania, D. Dubbeldam, T. J. H. Vlugt, J. M. Castillo, P. J. Merkling, and S. Calero, J. Phys. Chem. C 2008, 112, 27, 9976–9979.
-
 Carbon dioxide in zeolites (with and without sodium cations) https://doi.org/10.1021/jp810871f
Carbon dioxide in zeolites (with and without sodium cations) https://doi.org/10.1021/jp810871f"Transferable Force Field for Carbon Dioxide Adsorption in Zeolites", Almudena García-Sánchez†, Conchi O. Ania, José B. Parra, David Dubbeldam, Thijs J. H. Vlugt, Rajamani Krishna, and Sofía Calero, J. Phys. Chem. C 2009, 113, 20, 8814–8820
-
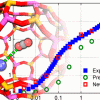 Carbon dioxide in zeolites with calcium cations https://doi.org/10.1021/jp507431c
Carbon dioxide in zeolites with calcium cations https://doi.org/10.1021/jp507431c"Insights on the Anomalous Adsorption of Carbon Dioxide in LTA Zeolites", A. Martin-Calvo, J. B. Parra, C. O. Ania, and S. Calero, J. Phys. Chem. C 2014, 118, 44, 25460–25467
-
 Oxygen, nitrogen and carbon monoxide in zeolites (with and without cations) https://doi.org/10.1039/c5cp03749b
Oxygen, nitrogen and carbon monoxide in zeolites (with and without cations) https://doi.org/10.1039/c5cp03749b"Transferable force fields for adsorption of small gases in zeolites", A. Martin-Calvo, J. J. Gutiérrez-Sevillano, J. B. Parra, C. O. Ania and S. Calero, Phys. Chem. Chem. Phys., 2015,17, 24048-24055
-
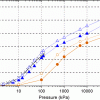 Water in pure silica zeolites https://doi.org/10.1080/08927020902865923
Water in pure silica zeolites https://doi.org/10.1080/08927020902865923"Evaluation of various water models for simulation of adsorption in hydrophobic zeolites", J.M. Castillo and D. Dubbeldam and T.J.H. Vlugt and B. Smit and S. Calero, Molecular Simulation, 2009, 35(12-13), 1067-1076
-
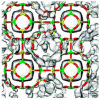 Water in zeolites with cations https://doi.org/10.1039/c3cp52910j
Water in zeolites with cations https://doi.org/10.1039/c3cp52910j"Water adsorption in hydrophilic zeolites: experiment and simulation", Juan Manuel Castillo, Juaquin Silvestre-Albero, Francisco Rodriguez-Reinoso, Thijs J. H. Vlugt and Sofia Calero, Phys. Chem. Chem. Phys., 2013,15, 17374-17382
-
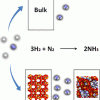 Ammonia in zeolites https://doi.org/10.1021/acs.jpcc.9b05366
Ammonia in zeolites https://doi.org/10.1021/acs.jpcc.9b05366"Improving Ammonia Production Using Zeolites", I. Matito-Martos, J. García-Reyes, A. Martin-Calvo, D. Dubbeldam, and S. Calero, J. Phys. Chem. C 2019, 123, 30, 18475–18481
-
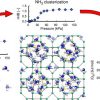 Ammonia in zeolites https://doi.org/10.1016/j.cej.2020.124062
Ammonia in zeolites https://doi.org/10.1016/j.cej.2020.124062"Role of hydrogen bonding in the capture and storage of ammonia in zeolites", I. Matito-Martos and A. Martin-Calvo and C.O. Ania and J.B. Parra and J.M. Vicent-Luna and S. Calero, Chemical Engineering Journal, 2020, 387, 124062
-
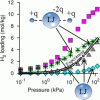 Hydrogen in zeolites (without cations) https://doi.org/10.1021/jp4037233
Hydrogen in zeolites (without cations) https://doi.org/10.1021/jp4037233"Insights on the Molecular Mechanisms of Hydrogen Adsorption in Zeolites", Kathryn S. Deeg, Juan José Gutiérrez-Sevillano, Rocío Bueno-Pérez, José B. Parra, Conchi O. Ania, Manuel Doblaré, and Sofía Calero, J. Phys. Chem. C 2013, 117, 27, 14374–14380
-
 Hydrogen in zeolites (with cations) https://doi.org/10.1021/acsami.0c20892
Hydrogen in zeolites (with cations) https://doi.org/10.1021/acsami.0c20892"In Silico Screening of Zeolites for High-Pressure Hydrogen Drying", Máté Erdős, Daan F. Geerdink, Ana Martin-Calvo, Evgeny A. Pidko, Leo J. P. van den Broeke, Sofia Calero, Thijs J. H. Vlugt, and Othonas A. Moultos, ACS Appl. Mater. Interfaces 2021, 13, 7, 8383–8394
-
 Hydrogen isotopes in zeolites https://doi.org/10.1021/acsami.9b02736
Hydrogen isotopes in zeolites https://doi.org/10.1021/acsami.9b02736"Molecular Sieves for the Separation of Hydrogen Isotopes", Julio Perez-Carbajo, José B. Parra, Conchi O. Ania, Patrick J. Merkling, and Sofia Calero, ACS Appl. Mater. Interfaces 2019, 11, 20, 18833–18840
-
 SO2 in zeolites https://doi.org/10.1039/c4cp00109e
SO2 in zeolites https://doi.org/10.1039/c4cp00109e"Zeolite screening for the separation of gas mixtures containing SO2, CO2 and CO", I. Matito-Martos, A. Martin-Calvo, J. J. Gutiérrez-Sevillano, M. Haranczyk, M. Doblare, J. B. Parra, C. O. Aniae and S. Calero, Phys. Chem. Chem. Phys., 2014,16, 19884-19893
-
 Sulphur hexafluoride in zeolites https://doi.org/10.1039/c5cp02407b
Sulphur hexafluoride in zeolites https://doi.org/10.1039/c5cp02407b"Zeolites for the selective adsorption of sulfur hexafluoride", I. Matito-Martos, J. Álvarez-Ossorio, J. J. Gutiérrez-Sevillano, M. Doblaré, A. Martin-Calvo and S. Calero, Phys. Chem. Chem. Phys., 2015,17, 18121-18130
-
 Lactic acid in zeolites https://doi.org/10.1021/jp504497t
Lactic acid in zeolites https://doi.org/10.1021/jp504497t"Enantiomeric Adsorption of Lactic Acid Mixtures in Achiral Zeolites", Ana Martin-Calvo, Sofía Calero, Johan A. Martens, and Titus S. van Erp, J. Phys. Chem. C 2014, 118, 27, 14991–14997
-
 Hydrocarbons (alkanes) in pure silica zeolites https://doi.org/10.1021/jp0376727
Hydrocarbons (alkanes) in pure silica zeolites https://doi.org/10.1021/jp0376727"United Atom Force Field for Alkanes in Nanoporous Materials", D. Dubbeldam, S. Calero, T. J. H. Vlugt, R. Krishna, T. L. M. Maesen, and B. Smit, J. Phys. Chem. B 2004, 108, 33, 12301–12313
-
 Hydrocarbons (alkanes) in pure silica zeolites https://doi.org/10.1103/PhysRevLett.93.088302
Hydrocarbons (alkanes) in pure silica zeolites https://doi.org/10.1103/PhysRevLett.93.088302"Force Field Parametrization through Fitting on Inflection Points in Isotherms", D. Dubbeldam, S. Calero, T. J. H. Vlugt, R. Krishna, T. L. M. Maesen, E. Beerdsen, and B. Smit, Phys. Rev. Lett. 2004, 93, 088302
-
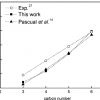 Hydrocarbons (alkanes) in pure silica zeolites https://doi.org/10.1021/jp064413j
Hydrocarbons (alkanes) in pure silica zeolites https://doi.org/10.1021/jp064413j"Evaluation of a New Force Field for Describing the Adsorption Behavior of Alkanes in Various Pure Silica Zeolites", Bei Liu, Berend Smit, and Sofia Calero, J. Phys. Chem. B 2006, 110, 41, 20166–20171
-
 Hydrocarbons (alkenes) in pure silica zeolites https://doi.org/10.1021/jp075809d
Hydrocarbons (alkenes) in pure silica zeolites https://doi.org/10.1021/jp075809d"A New United Atom Force Field for Adsorption of Alkenes in Zeolites", Bei Liu, Berend Smit, Fernando Rey, Susana Valencia, and Sofía Calero, J. Phys. Chem. C 2008, 112, 7, 2492–2498
-
 Hydrocarbons (alkenes) in pure silica zeolites https://doi.org/10.1021/jp101744k
Hydrocarbons (alkenes) in pure silica zeolites https://doi.org/10.1021/jp101744k"Analysis of the ITQ-12 Zeolite Performance in Propane−Propylene Separations Using a Combination of Experiments and Molecular Simulations", Juan José Gutiérrez-Sevillano, David Dubbeldam, Fernando Rey, Susana Valencia, Miguel Palomino, Ana Martín-Calvo, and Sofía Calero, J. Phys. Chem. C 2010, 114, 35, 14907–14914
-
 Hydrocarbons (alkanes) in zeolites with different Si/Al ratio and sodium cations https://doi.org/10.1021/ja0476056
Hydrocarbons (alkanes) in zeolites with different Si/Al ratio and sodium cations https://doi.org/10.1021/ja0476056"Understanding the Role of Sodium during Adsorption: A Force Field for Alkanes in Sodium-Exchanged Faujasites", Sofía Calero, David Dubbeldam, Rajamani Krishna, Berend Smit, Thijs J. H. Vlugt, Joeri F. M. Denayer, Johan A. Martens, and Theo L. M. Maesen, J. Am. Chem. Soc. 2004, 126, 36, 11377–11386
-
 Hydrocarbons (alkanes) in zeolites with different Si/Al ratio and sodium cations https://doi.org/10.1016/j.apsusc.2005.02.103
Hydrocarbons (alkanes) in zeolites with different Si/Al ratio and sodium cations https://doi.org/10.1016/j.apsusc.2005.02.103"Elucidating alkane adsorption in sodium-exchanged zeolites from molecular simulations to empirical equations", E.García-Pérez, I.M.Torréns, S.Lago, D.Dubbeldam, T.J.H.Vlugt, T.L.M.Maesen, B.Smit, R.Krishna, S.Calero, Applied Surface Science, 2005, 252(3), 716-722
-
 Hydrocarbons (alkanes) in zeolites with different Si/Al ratio and sodium cations https://doi.org/10.1016/s0167-2991(05)80453-7
Hydrocarbons (alkanes) in zeolites with different Si/Al ratio and sodium cations https://doi.org/10.1016/s0167-2991(05)80453-7"Molecular simulation of adsorption of n-alkanes in Na-MFI zeolites. Determination of empirical expressions", E.García-Pérez, I.M.Torréns, S.Lago, R.Krishna, B.Smit, S.Calero, Studies in Surface Science and Catalysis, 2005, 158, 1097-1104
-
 Hydrocarbons (alkanes) in zeolites with different Si/Al ratio and sodium and calcium cations https://doi.org/10.1021/jp064971y
Hydrocarbons (alkanes) in zeolites with different Si/Al ratio and sodium and calcium cations https://doi.org/10.1021/jp064971y"Influence of Cation Na/Ca Ratio on Adsorption in LTA 5A: A Systematic Molecular Simulation Study of Alkane Chain Length", E. García-Pérez, D. Dubbeldam, T. L. M. Maesen, and S. Calero, J. Phys. Chem. B 2006, 110, 47, 23968–23976
-
 Hydrocarbons (alkenes) zeolites with Na, Ca, cations https://doi.org/10.1016/j.cej.2019.122482
Hydrocarbons (alkenes) zeolites with Na, Ca, cations https://doi.org/10.1016/j.cej.2019.122482"π-Complexation for olefin/paraffin separation using aluminosilicates", A.Luna-Triguero, A.Sławek, R.Sánchez-de-Armas, J.J.Gutiérrez-Sevillano, C.O.Ania, J.B.Parra, J.M.Vicent-Luna, S.Calero, Chemical Engineering Journal, 2020, 380, 122482
-
 Hydrocarbons in zeolites with different Si/Al ratio and protons https://doi.org/10.1021/jp060174o
Hydrocarbons in zeolites with different Si/Al ratio and protons https://doi.org/10.1021/jp060174o"A Coarse-Graining Approach for the Proton Complex in Protonated Aluminosilicates", S. Calero, M. D. Lobato, E. García-Pérez, J. A. Mejías, S. Lago, T. J. H. Vlugt, T. L. M. Maesen, B. Smit, and D. Dubbeldam, J. Phys. Chem. B 2006, 110, 12, 5838–5841
-
 Hydrocarbons in zeolites with different Si/Al ratio and protons https://pubs.acs.org/doi/10.1021/acsanm.9b00416
Hydrocarbons in zeolites with different Si/Al ratio and protons https://pubs.acs.org/doi/10.1021/acsanm.9b00416"Enhancing the Water Capacity in Zr-Based Metal–Organic Framework for Heat Pump and Atmospheric Water Generator Applications", A. Luna-Triguero, A. Sławek, H. P. Huinink, T. J. H. Vlugt, A. Poursaeidesfahani, J. M. Vicent-Luna, and S. Calero, ACS Appl. Nano Mater. 2019, 2, 5, 3050–3059
-
 Benzene zeolites with cations https://doi.org/10.1016/j.cej.2020.125678
Benzene zeolites with cations https://doi.org/10.1016/j.cej.2020.125678"Exploiting the π-bonding for the separation of benzene and cyclohexane in zeolites", C.González-Galán, A.Luna-Triguero, J.M.Vicent-Luna, A.P.Zaderenko, A.Sławek, R.Sánchez-de-Armas, S.Calero, Chemical Engineering Journal, 2020, 398, 125678
Force fields implemented in RASPA for MOFs
-
 Carbon tetrachloride in CuBTC https://doi.org/10.1039/C0CC02194F
Carbon tetrachloride in CuBTC https://doi.org/10.1039/C0CC02194F"On the performance of Cu-BTC metal organic framework for carbon tetrachloride gas removal", Sofía Calero, Ana Martín-Calvo, Said Hamada and Elena García-Pérez, Chem. Commun., 2011,47, 508-510
-
 O2, N2, Ar and Carbon tetrachloride in CuBTC https://doi.org/10.1039/C1CP20168A
O2, N2, Ar and Carbon tetrachloride in CuBTC https://doi.org/10.1039/C1CP20168A"Effect of air humidity on the removal of carbon tetrachloride from air using Cu–BTC metal–organic framework", Ana Martín-Calvo, Elena García-Pérez, Almudena García-Sánchez, Rocío Bueno-Pérez, Said Hamada and Sofia Calero, Phys. Chem. Chem. Phys., 2011,13, 11165-11174
-
 Transferable set of point charges for ZIFs https://doi.org/10.1021/jp3107167
Transferable set of point charges for ZIFs https://doi.org/10.1021/jp3107167"Toward a Transferable Set of Charges to Model Zeolitic Imidazolate Frameworks: Combined Experimental–Theoretical Research", Juan José Gutiérrez Sevillano, Sofía Calero, Conchi O. Ania, José B. Parra, Freek Kapteijn, Jorge Gascon, and Said Hamad, J. Phys. Chem. C 2013, 117, 1, 466–471
-
 Hydrogen Sulfide in MOFs https://doi.org/10.1039/c3ra41682h
Hydrogen Sulfide in MOFs https://doi.org/10.1039/c3ra41682h"Adsorption of hydrogen sulphide on Metal-Organic Frameworks", Juan José Gutiérrez-Sevillano, Ana Martín-Calvo, David Dubbeldam, Sofía Calero and Said Hamad, RSC Adv., 2013,3, 14737-14749
-
 BTEX in MOFs https://doi.org/10.1021/jp411697z
BTEX in MOFs https://doi.org/10.1021/jp411697z"Selective Separation of BTEX Mixtures Using Metal–Organic Frameworks", Francisco D. Lahoz-Martín, Ana Martín-Calvo, and Sofía Calero, J. Phys. Chem. C 2014, 118, 24, 13126–13136
-
 CO in CuBTC https://doi.org/10.1021/jp211563e
CO in CuBTC https://doi.org/10.1021/jp211563e"Understanding Carbon Monoxide Capture Using Metal–Organic Frameworks", Ana Martín-Calvo, Francisco D. Lahoz-Martín, and Sofía Calero, J. Phys. Chem. C 2012, 116, 11, 6655–6663
-
 Acetylene in (Co, Fe) MOF-74 https://doi.org/10.1021/acsami.9b09010
Acetylene in (Co, Fe) MOF-74 https://doi.org/10.1021/acsami.9b09010"Acetylene Storage and Separation Using Metal–Organic Frameworks with Open Metal Sites", A. Luna-Triguero, J. M. Vicent-Luna, R. M. Madero-Castro, P. Gómez-Álvarez, and S. Calero, ACS Appl. Mater. Interfaces 2019, 11, 34, 31499–31507
-
 Alkane alkene in M-MOF-74 polarizable https://doi.org/10.1039/c8cp05750h
Alkane alkene in M-MOF-74 polarizable https://doi.org/10.1039/c8cp05750h"Potential of polarizable force fields for predicting the separation performance of small hydrocarbons in M-MOF-74", Tim M. Becker, Azahara Luna-Triguero, Jose Manuel Vicent-Luna, Li-Chiang Lin, David Dubbeldam, Sofia Calero and Thijs J. H. Vlugt, Phys. Chem. Chem. Phys., 2018,20, 28848-28859
-
 Alkane alkene in M-MOF-74 non-polarizable https://doi.org/10.1002/slct.201601095
Alkane alkene in M-MOF-74 non-polarizable https://doi.org/10.1002/slct.201601095"Effective Model for Olefin/Paraffin Separation using (Co, Fe, Mn, Ni)-MOF-74", Azahara Luna-Triguero, Jose Manuel Vicent-Luna, Tim M. Becker, Thijs J. H. Vlugt, David Dubbeldam, Paula Gómez-Álvarez, Sofia Calero, 2017, 2(2), 665-672
-
 Linear and branched alkene in Cu-BTC https://doi.org/10.1021/acs.jpcc.6b11808
Linear and branched alkene in Cu-BTC https://doi.org/10.1021/acs.jpcc.6b11808"Olefin/Paraffin Separation in Open Metal Site Cu-BTC Metal–Organic Framework", A. Luna-Triguero, J. M. Vicent-Luna, P. Gómez-Álvarez, and S. Calero, J. Phys. Chem. C 2017, 121, 5, 3126–3132
-
 Polarizable force fields https://doi.org/10.1039/c8cp05750h
Polarizable force fields https://doi.org/10.1039/c8cp05750h"Potential of polarizable force fields for predicting the separation performance of small hydrocarbons in M-MOF-74", Tim M. Becker, Azahara Luna-Triguero, Jose Manuel Vicent-Luna, Li-Chiang Lin, David Dubbeldam, Sofia Calero and Thijs J. H. Vlugt, Phys. Chem. Chem. Phys., 2018,20, 28848-28859
-
 Polarizable force fields http://dx.doi.org/10.1021/acs.jpcc.8b08639
Polarizable force fields http://dx.doi.org/10.1021/acs.jpcc.8b08639"Polarizable Force Field for CO2 in M-MOF-74 Derived from Quantum Mechanics", Tim M. Becker, Li-Chiang Lin, David Dubbeldam, and Thijs J. H. Vlugt, J. Phys. Chem. C 2018, 122, 42, 24488–24498
-
 Polarizable force fields http://dx.doi.org/10.1021/acs.jpcc.6b12052
Polarizable force fields http://dx.doi.org/10.1021/acs.jpcc.6b12052"Polarizable Force Fields for CO2 and CH4 Adsorption in M-MOF-74", Tim M. Becker, Jurn Heinen, David Dubbeldam, Li-Chiang Lin, and Thijs J. H. Vlugt, J. Phys. Chem. C 2017, 121, 8, 4659–4673
-
 Polarizable force fields http://dx.doi.org/10.1016/j.jocs.2015.08.010
Polarizable force fields http://dx.doi.org/10.1016/j.jocs.2015.08.010"Investigating polarization effects of CO2 adsorption in MgMOF-74", Tim M.Becker, David Dubbeldam, Li-Chiang Lin, Thijs J.H.Vlugt, 2016, 15, 86-94
Methods implemented in RASPA
-
 iRASPA and visualization https://doi.org/10.1080/08927022.2018.1426855
iRASPA and visualization https://doi.org/10.1080/08927022.2018.1426855"iRASPA: GPU-accelerated visualization software for materials scientists", David Dubbeldam, Sofia Calero, Thijs J. Vlugt, Molecular Simulation, 2018, 44(8), 653-676
-
 iRASPA and visualization https://doi.org/10.1016/j.coche.2019.02.001
iRASPA and visualization https://doi.org/10.1016/j.coche.2019.02.001"Highlights of (bio-)chemical tools and visualization software for computational science", David Dubbeldam,Jocelyne Vreede, Thijs J.H. Vlugt, Sofia Calero, 2019, 23, 1-13
-
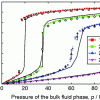 RASPA and Monte Carlo methods https://doi.org/10.1080/08927022.2015.1010082
RASPA and Monte Carlo methods https://doi.org/10.1080/08927022.2015.1010082"RASPA: molecular simulation software for adsorption and diffusion in flexible nanoporous materials", David Dubbeldam, Sofia Calero, Donald E. Ellis, Randall Q. Snurr, 2016, 42(2), 81-101
-
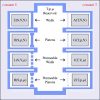 RASPA and Monte Carlo methods https://doi.org/10.1080/08927022.2013.819102
RASPA and Monte Carlo methods https://doi.org/10.1080/08927022.2013.819102"On the inner workings of Monte Carlo codes", David Dubbeldam, Ariana Torres-Knoop, K.S. Walton, 2013, 39(14-15), 1253-1292
-
 Parameterization of force fields https://doi.org/10.1002/adts.201900135
Parameterization of force fields https://doi.org/10.1002/adts.201900135"Design, Parameterization, and Implementation of Atomic Force Fields for Adsorption in Nanoporous Materials", David Dubbeldam, Krista S. Walton, Thijs J. H. Vlugt, Sofia Calero, Advanced Theory and Simulations, 2019, 2(11), 1900135
-
 Brick-CFCMC (Software for CFCMC in various ensembles) https://doi.org/10.1021/acs.jcim.0c00334
Brick-CFCMC (Software for CFCMC in various ensembles) https://doi.org/10.1021/acs.jcim.0c00334"Brick-CFCMC: Open Source Software for Monte Carlo Simulations of Phase and Reaction Equilibria Using the Continuous Fractional Component Method", Remco Hens, Ahmadreza Rahbari, Sebastián Caro-Ortiz, Noura Dawass, Máté Erdős, Ali Poursaeidesfahani, Hirad S. Salehi, Alper T. Celebi, Mahinder Ramdin, Othonas A. Moultos, David Dubbeldam, and Thijs J. H. Vlugt, J. Chem. Inf. Model. 2020, 60, 6, 2678–2682
-
 Brick-CFCMC (Software for CFCMC in various ensembles) https://doi.org/10.1021/acs.jcim.1c00652
Brick-CFCMC (Software for CFCMC in various ensembles) https://doi.org/10.1021/acs.jcim.1c00652"New Features of the Open Source Monte Carlo Software Brick-CFCMC: Thermodynamic Integration and Hybrid Trial Moves", H. Mert Polat, Hirad S. Salehi, Remco Hens, Dominika O. Wasik, Ahmadreza Rahbari, Frédérick de Meyer, Céline Houriez, Christophe Coquelet, Sofia Calero, David Dubbeldam, Othonas A. Moultos, and Thijs J. H. Vlugt, J. Chem. Inf. Model. 2021, 61, 8, 3752–3757
-
 CFCMC Review https://doi.org/10.1080/08927022.2020.1828585
CFCMC Review https://doi.org/10.1080/08927022.2020.1828585"Recent advances in the continuous fractional component Monte Carlo methodology", A. Rahbari, R. Hens, M. Ramdin, O. A. Moultos, D. Dubbeldam, T.J.H. Vlugt, Molecular Simulation, 2021, 47(10-11), 804-823
-
 Finite-size effectsof diffusion coefficients computed by MD https://doi.org/10.1080/08927022.2020.1810685
Finite-size effectsof diffusion coefficients computed by MD https://doi.org/10.1080/08927022.2020.1810685"Finite-size effects of diffusion coefficients computed from molecular dynamics: a review of what we have learned so far", Alper T. Celebi, Seyed Hossein Jamali, André Bardow, Thijs J. J. Vlugt, Othonas A. Moultos, Molecular Simulation, 2020, 47(10-11), 831-845
-
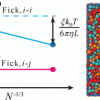 Finite-size effectsof diffusion coefficients computed by MD https://doi.org/10.1021/acs.jctc.0c00268
Finite-size effectsof diffusion coefficients computed by MD https://doi.org/10.1021/acs.jctc.0c00268"Generalized Form for Finite-Size Corrections in Mutual Diffusion Coefficients of Multicomponent Mixtures Obtained from Equilibrium Molecular Dynamics Simulation", Seyed Hossein Jamali, André Bardow, Thijs J. H. Vlugt, and Othonas A. Moultos, J. Chem. Theory Comput. 2020, 16, 6, 3799–3806
-
 Introduction to Molecular Simulation and Statistical Thermodynamics
Introduction to Molecular Simulation and Statistical Thermodynamics"Introduction to Molecular Simulation and Statistical Thermodynamics", Thijs J.H. Vlugt, Jan P.J.M. van der Eerden, Marjolein Dijkstra, Berend Smit, Daan Frenkel, 2009, https://homepage.tudelft.nl/v9k6y/imsst/index.html
-
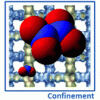 Reaction ensemble for nitrogen dioxide https://doi.org/10.1039/C7CP08017D
Reaction ensemble for nitrogen dioxide https://doi.org/10.1039/C7CP08017D"Adsorption equilibrium of nitrogen dioxide in porous materials", I. Matito-Martos, A. Rahbari, A. Martin-Calvo, D. Dubbeldam, T. J. H. Vlugt and S. Calero, Phys. Chem. Chem. Phys., 2018,20, 4189-4199
-
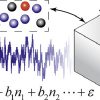 Computing the heat of adsorption for mixtures https://doi.org/10.1016/j.fluid.2020.112785
Computing the heat of adsorption for mixtures https://doi.org/10.1016/j.fluid.2020.112785"Multiple linear regression and thermodynamic fluctuations are equivalent for computing thermodynamic derivatives from molecular simulation", Ahmadreza Rahbaria, Tyler R. Josephson, Yangzesheng Sun, Othonas A. Moultos, David Dubbeldam, J. Ilja Siepmann, Thijs J.H.Vlugt, Fluid Phase Equilibria, 2020, 523, 112785
-
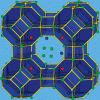 Computing the heat of adsorption for mixtures http://dx.doi.org/10.1021/ct700342k
Computing the heat of adsorption for mixtures http://dx.doi.org/10.1021/ct700342k"Computing the Heat of Adsorption using Molecular Simulations: The Effect of Strong Coulombic Interactions", T. J. H. Vlugt, E. García-Pérez, D. Dubbeldam, S. Ban∥, and S. Calero, J. Chem. Theory Comput. 2008, 4, 7, 1107–1118
Flexibility as used in RASPA
-
 Influence of framework flexibility for Zeolites https://doi.org/10.1021/acs.jpcc.0c08054
Influence of framework flexibility for Zeolites https://doi.org/10.1021/acs.jpcc.0c08054"Effects of Framework Flexibility on the Adsorption and Diffusion of Aromatics in MFI-Type Zeolites", Sebastián Caro-Ortiz, Erik Zuidema, Marcello Rigutto, David Dubbeldam, and Thijs J. H. Vlugt, J. Phys. Chem. C 2020, 124, 44, 24488–24499
-
 Influence of framework flexibility for Zeolites http://dx.doi.org/10.1021/jp0263931
Influence of framework flexibility for Zeolites http://dx.doi.org/10.1021/jp0263931"Influence of Framework Flexibility on the Adsorption Properties of Hydrocarbons in the Zeolite Silicalite", Thijs J. H. Vlugt and Merijn Schenk, J. Phys. Chem. B 2002, 106, 49, 12757–12763
-
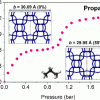 Influence of framework flexibility for Zeolites https://doi.org/10.1021/acs.langmuir.8b00628
Influence of framework flexibility for Zeolites https://doi.org/10.1021/acs.langmuir.8b00628"Stepped Propane Adsorption in Pure-Silica ITW Zeolite", Jung Gi Min, Azahara Luna-Triguero, Youngchul Byun, Salvador R. G. Balestra, Jose Manuel Vicent-Luna, Sofia Calero*, Suk Bong Hong*, and Miguel A. Camblor, Langmuir 2018, 34, 16, 4774–4779
-
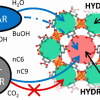 Influence of framework flexibility for MOFs https://doi.org/10.1021/acs.chemmater.8b01603
Influence of framework flexibility for MOFs https://doi.org/10.1021/acs.chemmater.8b01603"Gate-Opening Mechanism of Hydrophilic–Hydrophobic Metal–Organic Frameworks: Molecular Simulations and Quasi-Equilibrated Desorption", Andrzej Sławek, José Manuel Vicent-Luna, Bartosz Marszałek, Barbara Gil, Russell E. Morris, Wacław Makowski*, and Sofía Calero, Chem. Mater. 2018, 30, 15, 5116–5127
-
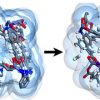 Influence of framework flexibility for MOFs https://doi.org/10.1002/chem.201801157
Influence of framework flexibility for MOFs https://doi.org/10.1002/chem.201801157"Phase Transition Induced by Gas Adsorption in Metal-Organic Frameworks", Azahara Luna-Triguero, Dr. Jose Manuel Vicent-Luna, Prof. Sofia Calero, Chemistry A European Journal, 2018, 24(34), 8530-8534
Simulations in the Bulk
-
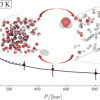 Hydrogen/water systems (bulk) https://doi.org/10.1021/acs.jced.9b00513
Hydrogen/water systems (bulk) https://doi.org/10.1021/acs.jced.9b00513"Solubility of Water in Hydrogen at High Pressures: A Molecular Simulation Study", Ahmadreza Rahbari, Jeroen Brenkman, Remco Hens, Mahinder Ramdin, Leo J. P. van den Broeke, Rogier Schoon, Ruud Henkes, Othonas A. Moultos, and Thijs J. H. Vlugt, J. Chem. Eng. Data 2019, 64, 9, 4103–4115
-
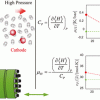 Hydrogen/water systems (bulk) https://doi.org/10.1021/acs.jced.1c00020
Hydrogen/water systems (bulk) https://doi.org/10.1021/acs.jced.1c00020"Effect of Water Content on Thermodynamic Properties of Compressed Hydrogen", Ahmadreza Rahbari, Julio C. Garcia-NavarroJulio C. Garcia-Navarro HyET Hydrogen BV, Westervoortsedijk 71K, 6827AV Arnhem, The Netherlands More by Julio C. Garcia-Navarro , Mahinder Ramdin, Leo J. P. van den Broeke, Othonas A. Moultos, David Dubbeldam, and Thijs J. H. Vlugt, J. Chem. Eng. Data 2021, 66, 5, 2071–2087
-
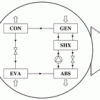 Ammonia-ionic liquid systems http://dx.doi.org/10.1021/acs.iecr.8b00442
Ammonia-ionic liquid systems http://dx.doi.org/10.1021/acs.iecr.8b00442"Absorption Refrigeration Cycles with Ammonia–Ionic Liquid Working Pairs Studied by Molecular Simulation", Tim M. Becker, Meng Wang, Abhishek Kabra, Seyed Hossein Jamali, Mahinder Ramdin, David Dubbeldam, Carlos A. Infante Ferreira, and Thijs J. H. Vlugt, Ind. Eng. Chem. Res. 2018, 57, 15, 5442–5452
-
 Water-Ionic liquid systems https://doi.org/10.1002/cphc.201501022
Water-Ionic liquid systems https://doi.org/10.1002/cphc.201501022"Aqueous Solutions of Ionic Liquids: Microscopic Assembly", Jose Manuel Vicent-Luna, David Dubbeldam, Paula Gómez-Álvarez, Sofia Calero, ChemPhysChem, 2016, 17(3), 380-386
-
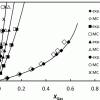 Gas solubility in ionic liquids (Osmotic ensemble) https://doi.org/10.1021/jp5080434
Gas solubility in ionic liquids (Osmotic ensemble) https://doi.org/10.1021/jp5080434"Solubility of the Precombustion Gases CO2, CH4, CO, H2, N2, and H2S in the Ionic Liquid [bmim][Tf2N] from Monte Carlo Simulations", Mahinder Ramdin, Sayee Prasaad Balaji, José Manuel Vicent-Luna, Juan José Gutiérrez-Sevillano, Sofía Calero, Theo W. de Loos, and Thijs J. H. Vlugt, J. Phys. Chem. C 2014, 118, 41, 23599–23604
-
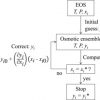 Gas solubility in ionic liquids (Osmotic ensemble) https://doi.org/10.1016/j.fluid.2015.09.041
Gas solubility in ionic liquids (Osmotic ensemble) https://doi.org/10.1016/j.fluid.2015.09.041"Computing bubble-points of CO2/CH4 gas mixtures in ionic liquids from Monte Carlo simulations", Mahinder Ramdina, Sayee Prasaad Balajia, José Manuel Vicent-Luna. Ariana Torres-Knoop, Qu Chena, David Dubbeldam, SofíaCalero, Theo W.de Loos, Thijs J.H.Vlugt, Fluid Phase Equilibria, 2016, 418, 100-107
-
 Gas solubility in ionic liquids (Osmotic ensemble) https://doi.org/10.1016/j.jocs.2015.09.002
Gas solubility in ionic liquids (Osmotic ensemble) https://doi.org/10.1016/j.jocs.2015.09.002"Solubilities of CO2, CH4, C2H6, and SO2 in ionic liquids and Selexol from Monte Carlo simulations", Mahinder Ramdina, Qu Chena, Sayee Prasaad Balajia, José Manuel Vicent-Luna, Ariana Torres-Knoop, David Dubbeldam, Sofía Calero, Theo W.de Loos, Thijs J.H.Vlugt, 2016, 15, 74-80
Applications in Zeolites
-
 Removing traces of water from hydrogen gas using zeolites https://doi.org/10.1021/acsami.0c20892
Removing traces of water from hydrogen gas using zeolites https://doi.org/10.1021/acsami.0c20892"In Silico Screening of Zeolites for High-Pressure Hydrogen Drying", Máté Erdős, Daan F. Geerdink, Ana Martin-Calvo, Evgeny A. Pidko, Leo J. P. van den Broeke, Sofia Calero, Thijs J. H. Vlugt, and Othonas A. Moultos, ACS Appl. Mater. Interfaces 2021, 13, 7, 8383–8394
-
 Xylene adsorption and isomerization in zeolites https://doi.org/10.1021/acs.jpcc.0c09411
Xylene adsorption and isomerization in zeolites https://doi.org/10.1021/acs.jpcc.0c09411"Competitive Adsorption of Xylenes at Chemical Equilibrium in Zeolites", Sebastián Caro-Ortiz, Erik Zuidema, Marcello Rigutto, David Dubbeldam, and Thijs J. H. Vlugt, J. Phys. Chem. C 2021, 125, 7, 4155–4174
-
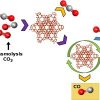 Syngas separation, CO purification https://doi.org/10.1016/j.cattod.2020.03.061
Syngas separation, CO purification https://doi.org/10.1016/j.cattod.2020.03.061"Enhancing separation efficiency in European syngas industry by using zeolites", A.Luna-Triguero, J.M.Vicent-Luna, M.J.Jansman, G.Zafeiropoulos, M.N.Tsampas, M.C.M.van de Sanden, H.N.Akse, S.Calero, Catalysis Today, 2021, 362, 113-121
-
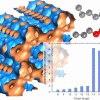 Window effect in zeolites https://doi.org/10.1021/acs.jpcc.5b05597
Window effect in zeolites https://doi.org/10.1021/acs.jpcc.5b05597"Understanding and Exploiting Window Effects for Adsorption and Separations of Hydrocarbons", A. Luna-Triguero, J. M. Vicent-Luna, D. Dubbeldam, P. Gómez-Álvarez, and S. Calero, J. Phys. Chem. C 2015, 119, 33, 19236–19243
-
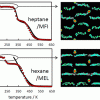 Isobaric adsorption of hydrocarbons in zeolites https://doi.org/10.1021/acs.jpcc.6b06957
Isobaric adsorption of hydrocarbons in zeolites https://doi.org/10.1021/acs.jpcc.6b06957"Adsorption of n-Alkanes in MFI and MEL: Quasi-Equilibrated Thermodesorption Combined with Molecular Simulations", Andrzej Sławek, José Manuel Vicent-Luna, Bartosz Marszałek, Salvador R. G. Balestra, Wacław Makowski, and Sofía Calero, J. Phys. Chem. C 2016, 120, 44, 25338–25350
-
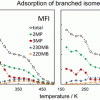 Isobaric adsorption of hydrocarbons in zeolites https://doi.org/10.1021/acs.jpcc.7b05347
Isobaric adsorption of hydrocarbons in zeolites https://doi.org/10.1021/acs.jpcc.7b05347"Quasi-Equilibrated Thermodesorption Combined with Molecular Simulation for Adsorption and Separation of Hexane Isomers in Zeolites MFI and MEL", Andrzej Sławek, José Manuel Vicent-Luna, Bartosz Marszałek, Wacław Makowski, and Sofía Calero, J. Phys. Chem. C 2017, 121, 35, 19226–19238
-
 Isobaric adsorption of hydrocarbons in zeolites https://doi.org/10.1021/acs.jpcc.7b08927
Isobaric adsorption of hydrocarbons in zeolites https://doi.org/10.1021/acs.jpcc.7b08927"Ordering of n-Alkanes Adsorbed in the Micropores of AlPO4-5: A Combined Molecular Simulations and Quasi-Equilibrated Thermodesorption Study", Andrzej Sławek, José Manuel Vicent-Luna, Bartosz Marszałek, Wacław Makowski, and Sofía Calero, J. Phys. Chem. C 2017, 121, 45, 25292–25302
-
 Isobaric adsorption of hydrocarbons in zeolites https://doi.org/10.1002/cphc.201800968
Isobaric adsorption of hydrocarbons in zeolites https://doi.org/10.1002/cphc.201800968"Adsorption of Cyclohexane in Pure Silica Zeolites: High-Throughput Computational Screening Validated by Experimental Data", Andrzej Sławek, Karolina Grzybowska, José Manuel Vicent-Luna, Wacław Makowski, Sofía Calero, ChemPhysChem, 2018, 19(24), 3364-3371
-
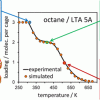 Isobaric adsorption of hydrocarbons in zeolites https://doi.org/10.1021/acs.jpcc.9b07907
Isobaric adsorption of hydrocarbons in zeolites https://doi.org/10.1021/acs.jpcc.9b07907"Adsorption of Alkanes in Zeolites LTA and FAU: Quasi-Equilibrated Thermodesorption Supported by Molecular Simulations", Andrzej Sławek*, José Manuel Vicent-Luna, Karolina Ogorzały, Susana Valencia, Fernando Rey, Wacław Makowski, and Sofía Calero, J. Phys. Chem. C 2019, 123, 49, 29665–29678
-
 Propane & Propylene in zeolite ITQ-12 https://doi.org/10.1021/jp101744k
Propane & Propylene in zeolite ITQ-12 https://doi.org/10.1021/jp101744k"Analysis of the ITQ-12 Zeolite Performance in Propane−Propylene Separations Using a Combination of Experiments and Molecular Simulations", Juan José Gutiérrez-Sevillano, David Dubbeldam, Fernando Rey, Susana Valencia, Miguel Palomino, Ana Martín-Calvo, and Sofía Calero, J. Phys. Chem. C 2010, 114, 35, 14907–14914
Applications in MOFs
-
 MOFs for adsorption driven heat pumps and chillers http://dx.doi.org/10.1021/acsami.8b09343
MOFs for adsorption driven heat pumps and chillers http://dx.doi.org/10.1021/acsami.8b09343"In Silico Screening of Metal–Organic Frameworks for Adsorption-Driven Heat Pumps and Chillers", Máté Erdős, Martijn F. de Lange, Freek Kapteijn, Othonas A. Moultos, and Thijs J. H. Vlugt, ACS Appl. Mater. Interfaces 2018, 10, 32, 27074–27087
-
 MOFs for adsorption driven heat pumps and chillers http://dx.doi.org/10.1021/acs.langmuir.5b03272
MOFs for adsorption driven heat pumps and chillers http://dx.doi.org/10.1021/acs.langmuir.5b03272"Metal–Organic Frameworks in Adsorption-Driven Heat Pumps: The Potential of Alcohols as Working Fluids", Martijn F. de Lange, Benjamin L. van Velzen, Coen P. Ottevanger, Karlijn J. F. M. Verouden, Li-Chiang Lin, Thijs J. H. Vlugt, Jorge Gascon, and Freek Kapteijn, Langmuir 2015, 31, 46, 12783–12796
-
 MOFs for adsorption driven heat pumps and chillers http://dx.doi.org/10.1021/acs.chemrev.5b00059
MOFs for adsorption driven heat pumps and chillers http://dx.doi.org/10.1021/acs.chemrev.5b00059"Adsorption-Driven Heat Pumps: The Potential of Metal–Organic Frameworks", Martijn F. de Lange, Karlijn J. F. M. Verouden, Thijs J. H. Vlugt, Jorge Gascon, and Freek Kapteijn, Chem. Rev. 2015, 115, 22, 12205–12250
-
 MOFs for adsorption driven heat pumps and chillers http://dx.doi.org/10.1039/C5CE00789E
MOFs for adsorption driven heat pumps and chillers http://dx.doi.org/10.1039/C5CE00789E"Manufacture of dense CAU-10-H coatings for application in adsorption driven heat pumps: optimization and characterization", M. F. de Lange, T. Zeng, T. J. H. Vlugt, J. Gascona and F. Kapteijn, CrystEngComm, 2015,17, 5911-5920
-
 MOFs for adsorption driven heat pumps and chillers https://doi.org/10.1021/acsanm.9b00416
MOFs for adsorption driven heat pumps and chillers https://doi.org/10.1021/acsanm.9b00416"Enhancing the Water Capacity in Zr-Based Metal–Organic Framework for Heat Pump and Atmospheric Water Generator Applications", A. Luna-Triguero, A. Sławek, H. P. Huinink, T. J. H. Vlugt, A. Poursaeidesfahani, J. M. Vicent-Luna, and S. Calero, ACS Appl. Nano Mater. 2019, 2, 5, 3050–3059
-
 Alcohol capture https://doi.org/10.1021/acs.jpcc.9b05508
Alcohol capture https://doi.org/10.1021/acs.jpcc.9b05508"Adsorption of Light Alcohols in a High Hydrophobic Metal Azolate Framework", R. M. Madero-Castro, J. M. Vicent-Luna*, and S. Calero, J. Phys. Chem. C 2019, 123, 39, 23987–23994
-
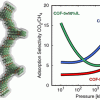 Ionic liquid/MOFs for enhanced CO2 separation https://doi.org/10.1021/acs.jpcc.6b05233
Ionic liquid/MOFs for enhanced CO2 separation https://doi.org/10.1021/acs.jpcc.6b05233"Storage and Separation of Carbon Dioxide and Methane in Hydrated Covalent Organic Frameworks", J. M. Vicent-Luna, A. Luna-Triguero, and S. Calero, J. Phys. Chem. C 2016, 120, 41, 23756–23762
-
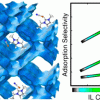 Ionic liquid/MOFs for enhanced CO2 separation https://doi.org/10.1021/acsami.8b11842
Ionic liquid/MOFs for enhanced CO2 separation https://doi.org/10.1021/acsami.8b11842"Role of Ionic Liquid [EMIM]+[SCN]− in the Adsorption and Diffusion of Gases in Metal–Organic Frameworks", Jose Manuel Vicent-Luna, Juan Jose Gutiérrez-Sevillano, Said Hamad, Juan Anta, and Sofia Calero, ACS Appl. Mater. Interfaces 2018, 10, 35, 29694–29704
-
 Water in UiO-66 https://doi.org/10.1016/j.micromeso.2021.111555
Water in UiO-66 https://doi.org/10.1016/j.micromeso.2021.111555"Water adsorption in ideal and defective UiO-66 structures", GabrielaJ ajko, Juan José Gutiérrez-Sevillano, Andrzej Sławek, Monika Szufla, Paweł Kozyra, Dariusz Matoga, Wacław Makowski, Sofia Calero, Microporous and Mesoporous Materials, 2022, 330, 111555
-
 Water in UiO-66 https://doi.org/10.1002/chem.202102181
Water in UiO-66 https://doi.org/10.1002/chem.202102181"Carbon Dioxide Capture Enhanced by Pre-Adsorption of Water and Methanol in UiO-66", Gabriela Jajko, Paweł Kozyra, Juan José Gutiérrez-Sevillano, Wacław Makowski, Sofia Calero, Chemistry an European Journal, 2021, 27(59),14653-14659
-
 Alcohols, benzene and water in MAF-6 https://doi.org/10.1002/adts.201900112
Alcohols, benzene and water in MAF-6 https://doi.org/10.1002/adts.201900112"Using Aliphatic Alcohols to Tune Benzene Adsorption in MAF-6", Ana Martin-Calvo, Juan Jose Gutierrez-Sevillano, David Dubbeldam, Sofia Calero, Advanced Theory and Simulations, 2019, 2(11), 1900112
-
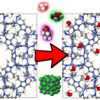 Alcohols, benzene and water in MAF-6 https://doi.org/10.1016/j.seppur.2021.119422
Alcohols, benzene and water in MAF-6 https://doi.org/10.1016/j.seppur.2021.119422"Modifying the hydrophobic nature of MAF-6", Juan José Gutiérrez-Sevillano, Ana Martin-Calvo, David Dubbeldam, Sofia Calero, Separation and Purification Technology, 2021, 277, 119422
